Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules and Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules prescription drug blister packages
Unknown Brand
Product Images
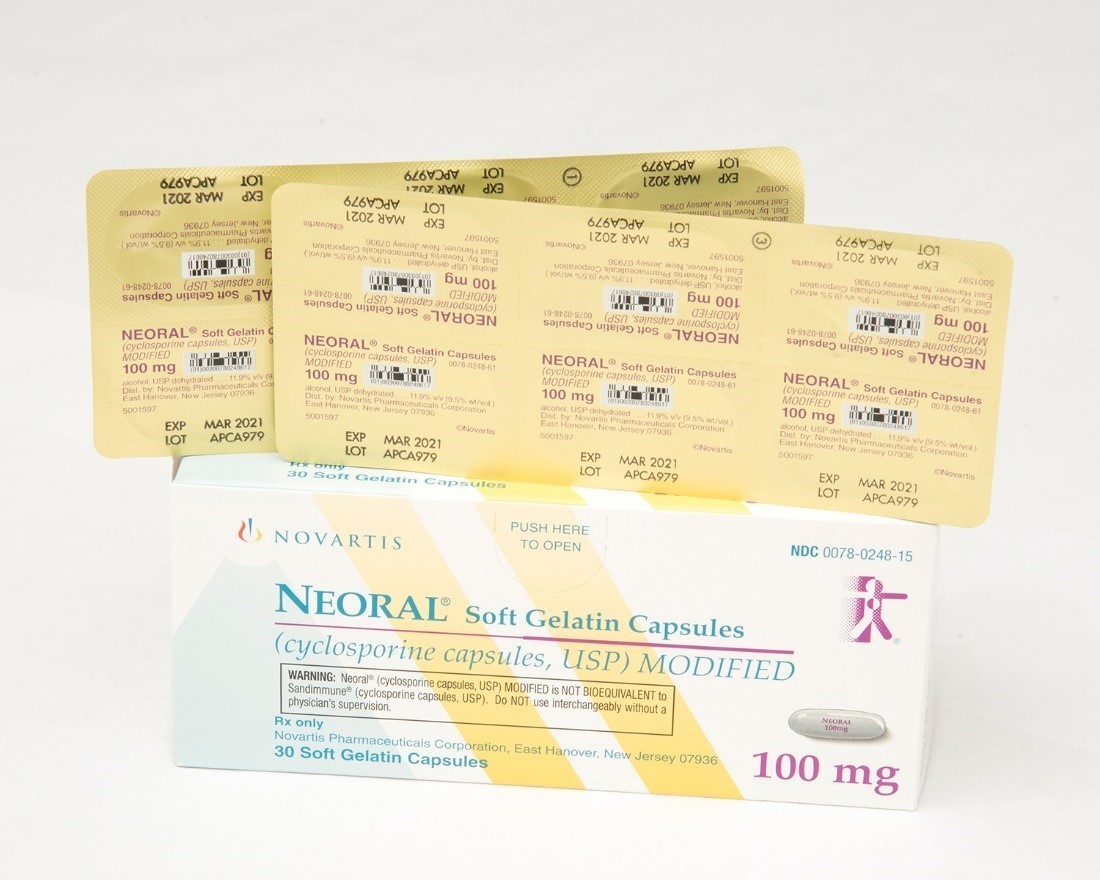
Recalled Neoral® 100 mg soft gelatin capsules
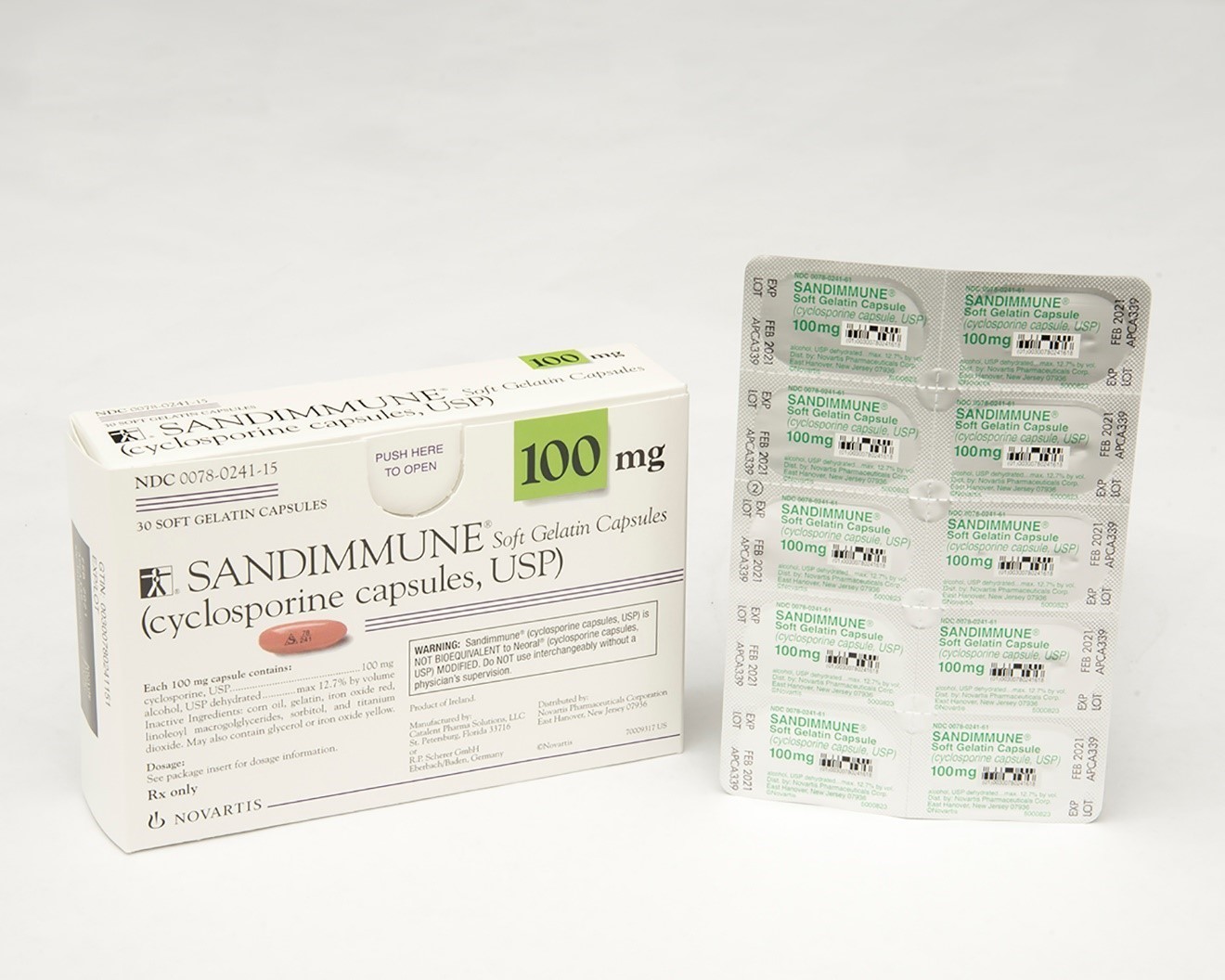
Recalled Sandimmune® 100 mg soft gelatin capsules
Why is it being recalled? (Hazard)
The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act (PPPA), posing a risk of poisoning if the contents are swallowed by young children.
What should I do? (Remedy)
Consumers should immediately secure the product out of the sight and reach of children and contact the firm to request a free child-resistant pouch in which to store the blister package medications. Consumers can continue to use the medication as directed. The child-resistant pouches should be used to store these medications until new child-resistant blister packaging is available.
Consumer Contact
Novartis toll-free at 866-629-6182 from 8 a.m. to 8 p.m. ET daily, email at [email protected] or online at www.pharma.us.novartis.com and in the top navigation of the page go to the News tab and click on Statements, or visit https://www.pharma.us.novartis.com/news/statements/corrective-action-certain-100-mg-sandimmune-and-neoral-blister-packages-us for more information.
Product Description
This recall involves blister packages of prescription medications Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules and Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules from Novartis. Packages of Sandimmune 100 mg contain three blister cards with ten soft gelatin capsules per card and packages of Neoral 100 mg contain five blister cards with six soft gelatin capsules per card. The recalled blister packages have "Novartis," the name of the medication, dosage, NDC, lot number and expiration date on the outer package and on the blister cards. Only 100 mg doses of these medications with the following NDC and lot numbers and expiration dates are included in this recall: Recalled Prescription Drugs NDC Numbers Lot Numbers Expiration Date Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules 0078-0241-15 0078-0241-61 APCA136 APCA339 APCA793 APCC238 09/2020 02/2021 01/2022 07/2022 Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules 0078-0248-15 0078-0248-61 APCA437 APCA979 07/2020 03/2021
Where was it sold?
Clinics and pharmacies nationwide as a prescribed medicine from March 2018 through March 2020, at prices varying based on quantities prescribed, health insurance terms, and other factors.
Latest Recall Updates
Unknown Brand
Huaker Magnetic Balls and Rods Sets
February 19, 2026
Unknown Brand
JJGoo LED Balloon Lights
February 19, 2026
Joly's LLC, of Orlando, Florida
Joly's 80% Vinegar (32 oz, pack of two)
February 19, 2026
Meijer Distribution, Inc., of Grand Rapids, Michigan
Meijer one-piece footed 12-, 18-and 24-month children's sleepwear
February 19, 2026
Unknown Brand
Prismatic 3D Prints Book Nooks with Lights
February 19, 2026
Unknown Brand
SAMIT Youth Multi-Purpose Helmets
February 19, 2026
Quick Facts
Did you buy this?
Stop using the product immediately. Check the "Remedy" section for refund or repair instructions.