NumbSkin pain relief cream with lidocaine
SeeNext Venture Ltd., of Blaine, Wash.
Product Images
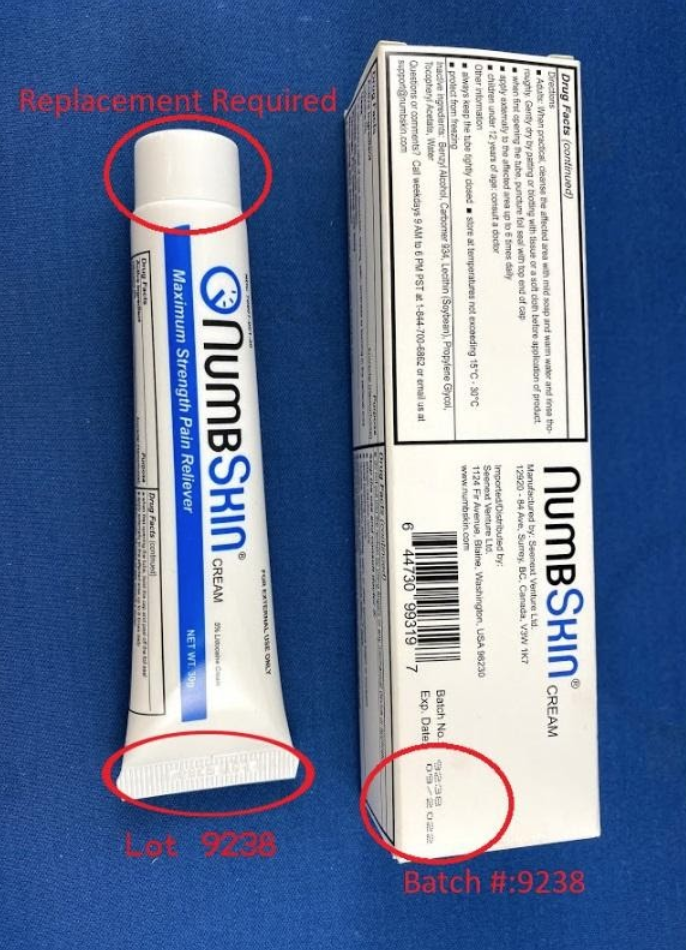
Recalled NumbSkin pain relief cream
Why is it being recalled? (Hazard)
The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain relieving cream contains lidocaine, posing a risk of poisoning to young children if they put it on their skin or ingest it.
What should I do? (Remedy)
Consumers should immediately store the pain relief cream in a safe location out of reach of children and contact SeeNext Venture for instructions on how to dispose or return it and to receive a free replacement similar product with a child-resistant cap. Amazon is contacting all known purchasers directly.
Consumer Contact
SeeNext Venture toll-free at 844-700-6862 from 9 a.m. to 6 p.m. PT Monday through Saturday, email at [email protected] or online at www.numbskin.com and click on Recall at the home page for more information.
Product Description
This recall involves NumbSkin pain relief cream with 5% lidocaine. The topical anesthetic cream was sold in 30 grams in a white with blue tube. NumbSkin is printed on the tube. Lot 9238 and a date code of 10/2019 through 09/2020 in a MM/YYYY format is embossed on the tub's thin end. Batch number 9238 is printed on the product packaging.
Where was it sold?
Online at Amazon.com from October 2019 through February 2020 for about $25.
Latest Recall Updates
Unknown Brand
Huaker Magnetic Balls and Rods Sets
February 19, 2026
Unknown Brand
JJGoo LED Balloon Lights
February 19, 2026
Joly's LLC, of Orlando, Florida
Joly's 80% Vinegar (32 oz, pack of two)
February 19, 2026
Meijer Distribution, Inc., of Grand Rapids, Michigan
Meijer one-piece footed 12-, 18-and 24-month children's sleepwear
February 19, 2026
Unknown Brand
Prismatic 3D Prints Book Nooks with Lights
February 19, 2026
Unknown Brand
SAMIT Youth Multi-Purpose Helmets
February 19, 2026
Quick Facts
Did you buy this?
Stop using the product immediately. Check the "Remedy" section for refund or repair instructions.