Product Images

Recalled bottle of wintergreen oil
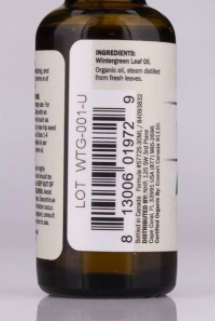
Location of Lot code and UPC code on recalled bottle
Why is it being recalled? (Hazard)
The products contain the substance methyl salicylate which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the product is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
What should I do? (Remedy)
Consumers should store the product in a safe location out of reach of children and contact Natural Health Partners for a free replacement child resistant cap or a full refund. Natural Health Partners is contacting all known purchasers directly about the recall.
Consumer Contact
Natural Health Partners toll-free at 877-985-2696 from 8 a.m. to 9 p.m. ET Monday through Friday and 9 a.m. to 6 p.m. ET Saturday through Sunday, email at [email protected] or online at www.shopmercola.com and click on ‘HELP’ at the top right corner of the page for more information.
Product Description
This recall involves Dr. Mercola Wintergreen Essential Oil in a 1 fl.oz (30 mL) amber glass bottles with black caps. The Lot codes for the recalled products are WTG-001-T and WTG-001-U, the UPC code is 813006019729. The Lot codes are printed on the product label above the UPC code.
Where was it sold?
Online at www.shop.mercola.com from December 2017 through August 2019 for about $30.
Latest Recall Updates
Unknown Brand
Huaker Magnetic Balls and Rods Sets
February 19, 2026
Unknown Brand
JJGoo LED Balloon Lights
February 19, 2026
Joly's LLC, of Orlando, Florida
Joly's 80% Vinegar (32 oz, pack of two)
February 19, 2026
Meijer Distribution, Inc., of Grand Rapids, Michigan
Meijer one-piece footed 12-, 18-and 24-month children's sleepwear
February 19, 2026
Unknown Brand
Prismatic 3D Prints Book Nooks with Lights
February 19, 2026
Unknown Brand
SAMIT Youth Multi-Purpose Helmets
February 19, 2026
Quick Facts
Did you buy this?
Stop using the product immediately. Check the "Remedy" section for refund or repair instructions.